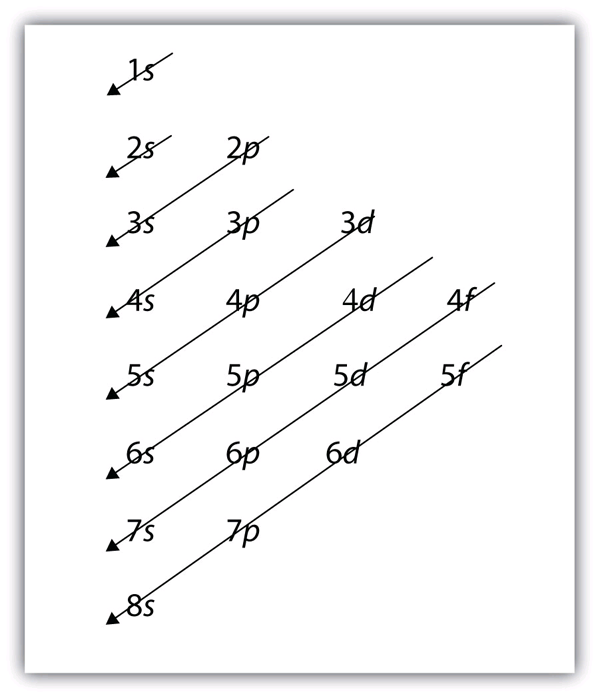
Just do subshell configuration for it:
Table:
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
s subshell has a max of 2 electrons
p subshell has a max of 6 electrons
d subshell has a max of 10 electrons
Then you just move diagonally (right to left) through the subshell table until you get to the number electrons needed. Since you are doing noble gas configuration, we will first start with Argon.
So finding the subshell configuration for Argon(18) is: 1s2 2s2 2p6 3s2 3p6 -- the number before the subshell tells you which main shell it is in and the second number tells you how many electrons in the subshell. if you add up all of the second numbers you will get 18, so we are finished with Argon.
This leaves us with:
V[Ar]. Now we just need to finish the subshell configuration until we reach 23 electrons
1s2 2s2 2p6 3s2 3p6 4s2 3d3 - This completes it and it leaves us with 2-8-11-2 as the structure for V :
Code
k = 2 = 1s2
l = 8 = 2s2 2p6
m = 11 = 3s2 3p6 3d3
n = 2 = 4s2
The noble gas configuration would be:
V[Ar]3d3 4s2
How you move through the subshell table in case it was confusing:
 This post was edited by kasey21 on Feb 18 2019 05:15pm
This post was edited by kasey21 on Feb 18 2019 05:15pm