Hey, I have a few questions on some of the problems for my chem homework and any help on any of them would be greatly appreciated

I tried to make it look as pretty as I could but it is still a little messy sry >.<
1) In an oxyacetylene welding torch, acetylene (C2H2) burns in pure oxygen with a very hot flame. How many grams of oxygen gas are required to react with 56.25 g of acetylene?
C2H2 + O2 CO2 + H2O (Hint: This isn't balanced)
2) Calculate the mass, in grams, of 0.324 moles of iodine pentafluoride.
These ones are multiple choice:
3) If I take an aspirin (C9H8O4) tablet containing 325 mg of aspirin, how many molecules of aspirin will I consume?
1. 3.52 x 1025 molecules
2. 3.00 x 10-27 molecules
3. 1.09 x 1024 molecules
4. 1.09 x 1021 molecules
4 ) ______ are in 10.0 moles of C10H 8.
1. 8.00 moles of hydrogen
2. 4.82 x 1025 atoms of hydrogen
3. 6.022 x 1024 atoms of carbon
4. 10.0 moles of carbon
5. 4.82 x 1024 atoms of hydrogen
5) What precipitate is formed in the reaction of aqueous Pb(NO3)2 with aqueous KI?
1. PbI2
2. KI
3. No precipitate is formed in this reaction.
4. PbI
6) Natural gas methane (CH4) burns in oxygen to yield water and carbon dioxide (CO2) as shown below:
CH4 + 2 O2
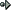
CO2 + 2 H2O
How many moles of carbon dioxide are produced from 3.8 moles of oxygen?
1. 3.8 mol
2. 7.6 mol
3. 2 mol
4. 1.9 mol
7) Which of the following statements is not true?
1. 10.00 moles of water contain 6.022 x 1024 molecules of water.
2. 10.00 moles of water and 2.000 moles of butane (C4H10) contain the same number of hydrogen atoms.
3. 10.00 moles of water and 10.00 moles of H2 contain the same number of molecules.
4. 10.00 moles of water contain 18.02 grams of water.
5. 10.00 moles of water and 5.000 moles of CO2 contain the same number of oxygen atoms.
8 ) When ammonia gas [NH3(g)] is passed over hot sodium, hydrogen gas is released and sodium amide, (NaNH2), is formed as a solid product. Write a balanced chemical equation for this process and be sure to indicate the state of each compound.
1. NH3(g) + Na(s)
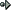
NaNH2(s) + H2(g)
2. 2 NH3(g) + 2 Na(s)
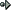
2 NaNH2(s) + H2(l)
3. 2 NH3 + 2 Na
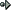
2 NaNH2 + H2
4. 2 NH3 + Na
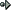
2 NaNH2 + H2
5. 2 NH3(g) + 2 Na(s)
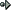
2 NaNH2(s) + H2(g)
9) If I add 5.00 g of sucrose (C12H22O11) to my cup of coffee, how many moles of sucrose did I add?
1. 68.4 moles
2. 0.172 mole
3. 1710 moles
4. 0.0146 moles
5. 0.014620 moles
10) When I metabolize 50.0 g of sucrose, how many grams of CO2 do I produce?
C12H22O11 + O2
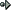
H2O + CO2 (Hint: This isn't balanced...)
1. 600 g
2. 0.536 g
3. 77.2 g
4. 6.43 g